Motor Protein Biophysics
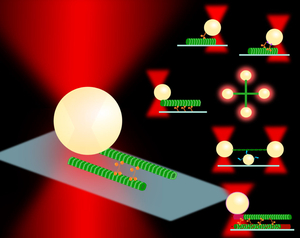
Biological motors are essential to sustain life. These proteins convert chemical energy into mechanical work through conformational changes from ATP hydrolysis. The kinesin family of motors uses this mechanical energy to walk hand-over-hand along highly conserved microtubule (MT) tracks to transport cargo through the cell and aid in cell division. The myosin family of motors associate with actin to transport cargo and generate the forces necessary for muscle contraction. Deficiencies in these instrumental proteins have been linked to many diseases, such as but not limited to Alzheimer’s disease in the case of transport kinesin in neurons and hypertrophic cardiomyopathy with myosin that causes heart hypercontracility. They are also the target of cancer therapies to aid in halting uncontrolled cell growth.
Single molecule studies using techniques such as optical trapping and fluorescence microscopy have been essential in evaluating the mechanistic behavior of molecular motors. Measurements can yield information that is overshadowed in bulk experiments, such as bond lifetimes, dissociation kinetics, step sizes, dwell times, and stall forces, among others, on the scale of piconewton (pN) forces and nanometer (nm) displacements. In these optical trapping assays, a micron-sized polystyrene bead is typically used as a handle to examine single molecules that are diluted and attached via various functionalization techniques. The bead is then trapped by a tightly focused laser beam, and any scattering due to displacement from the beam center is detected. The beam acts as a Hookean spring, so these displacements can be translated into force measurements by knowing the trap stiffness (or spring constant) from calibrations.
Synergy of Engineered Cytoskeletal Assemblies
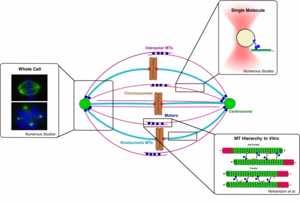
Traditional motor protein experiments involve a single molecule interacting with or moving along a single substrate. While this yields important information that cannot be attained from bulk experiments, the assay conditions do not accurately represent physiological conditions. The cell environment has structures of various hierarchies, from filament bundles to the dynamic mitotic spindle assembly to muscle contraction.
Therefore, reconstituting and probing systems at higher levels of complexity in vitro would yield more physiologically relevant data and reveal ensemble mechanics of motors, crosslinkers, and more complex combinations at the molecular level that give rise to large-scale cellular tasks. In addition, these assemblies could be used as diagnostic tools for evaluating the effects of mutated proteins or drugs on system performance.
Mechanics and Viscoelastic Characterization of Cytoskeletal Hierarchical Structures

Many in vitro biophysical assays, such as fluorescence and optical trapping experiments, are assembled on a glass coverslip and built from the bottom up in order to corroborate mechanistic information in bulk or cell-level studies. However, there is little known about the mechanisms of assembly and viscoelastic characteristics of these in vitro assemblies. Understanding these properties is foundational in interpreting biophysical information in the context of its cellular environment, and our approach is to use a quartz crystal microbalance with dissipation (QCM-D) to probe these interactions. We can further investigate how motor activation and synergy affect local viscoelastic properties and how those force signals and vibrations propagate throughout the cytoskeletal network.
Physical Properties of Cytoskeletal Building Blocks
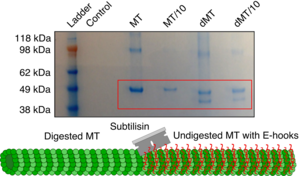
Individual components or domains of proteins have specific sequences that are essential for their function. Modulating even a single amino acid side group can alter the affinity of a protein for its substrate. Investigating the biophysical chemistry of motor protein/cytoskeletal filament interactions using spectroscopic methods can reveal the foundational principles of how these systems function.
Funding
The Reinemann Lab is grateful for past and current funding from the following sources:
National Institutes of Health
NIGMS R35 ESI MIRA Award (R35GM147050)
NIBIB ESTEEMED (R25EB036412)
National Science Foundation
NSF REU Site: Ole Miss Nanoengineering Summer REU Program (EEC 2148764)
NSF CAREER Award (BMMB 2439729)
American Heart Association
AHA Career Development Award (848586)
University of Mississippi
UM Summer Undergraduate Research Group Grant
UM Start-Up Funds
Sally McDonnell Barksdale Honors College
Honors Thesis Student Grants
NASA Mississippi Space Grant Consortium
Mississippi Space Grant Consortium Grant for NASA-Related Research NNX15AH78H
American Chemical Society
American Chemical Society Innovative Project Grant
Prior to appointment at UM:
National Science Foundation Graduate Research Fellowship (1445197)
Barry M. Goldwater Scholarship











