Research Sponsors
- National Institute of General Medical Sciences (R01GM127267, P41GM103390, R01GM096049A, P20GM104932)
- National Science Foundation Division of Chemistry (1608685)
- GenNext Technologies, Inc.
- The University of Mississippi
Hydroxyl Radical Protein Footprinting
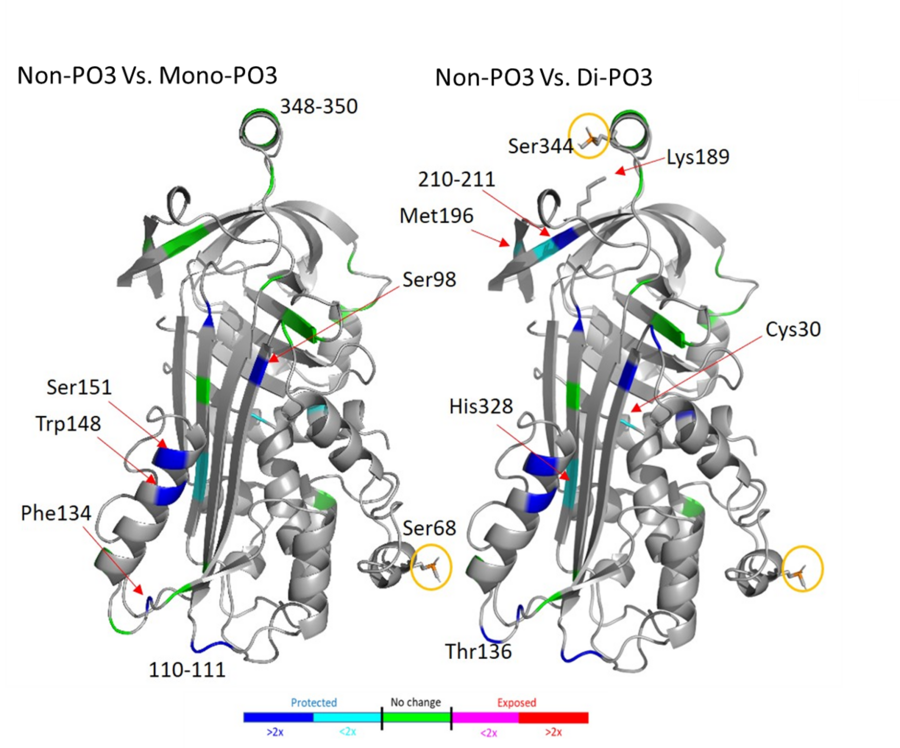
One of the major projects in our group is the development and application of new methods for studying protein structure, the most notable of which is hydroxyl radical protein footprinting. Hydroxyl radical protein footprinting (HRPF) coupled with liquid chromatography-mass spectrometry (LC-MS) has become an increasingly popular structural analysis technique to probe changes in protein topography, offering high labeling efficiency, stable modification products, and a flexible detection scheme. A protein is exposed to a short-lived concentration of hydroxyl radicals freely diffusing in solution, which oxidize the amino acid side chains of the protein. Amino acid side chains are oxidized at a rate based on their inherent reactivity and the accessibility to the hydroxyl radical diffusing in solution. By comparing the same protein in two or more conformational states (e.g., ligand-free versus ligand-bound), semi-quantitative measurements of changes in the protein topography can be made. Because of its flexibility and labeling efficiency, HRPF has been successfully used to measure protein folding, protein conformational change, protein-protein interactions, and protein-ligand binding surfaces in various proteins.

Dr. Sharp has worked to help develop a safe, benchtop ultra-fast method for generating these radicals, known as Flash Oxidation (FOX). FOX was invented by GenNext Technologies, Inc. by a team of researchers led by Joshua Sharp and Scot Weinberger with the aim of developing a turnkey solution for researchers seeking to bring HRPF to their laboratories. In the Sharp Lab at the University of Mississippi, we use and modify the FOX system to develop new associated footprinting technologies to push the boundaries of what can be measured.


A major focus of our group is developing methods for using HRPF topography data to create experimentally-informed computational models of protein structure. We were the first group to create a blinded, independently-confirmed HRPF-constrained model of a protein of unknown structure. We currently are working with a collaborator to develop methods to use HRPF to inform deep learning-based models of protein structure for challenging systems.

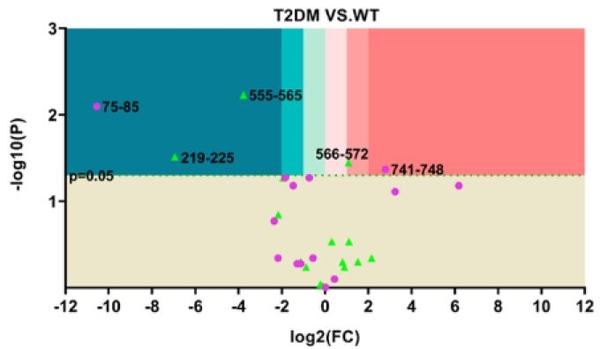
We have worked to combine HRPF inline with liquid chromatography to allow us to characterize individual conformers from within dynamic and complex mixtures of conformers and proteoforms. This has allowed us to tackle problems like the structural consequences of protein phosphorylation, as well as look at complex protein:polysaccharide complexes. Ongoing work is applying this technology towards other complex and dynamic protein systems to improve structural characterization of these challenging systems.
Radical Protein Footprinting in Mammalian Tissue
Complex Carbohydrate Structure
Complex carbohydrates are an understudied category of biomolecule, involved in most areas of cell-cell recognition, cellular signaling and cell development and disease. One of the major reasons complex carbohydrates are not as well understood as other biomolecules is the difficulties in studying these complex molecules. The Sharp Lab is active in the development of new technologies to approach some of the most challenging facets of complex carbohydrate structural biology: glycosaminoglycan structure and carbohydrate:protein interaction motifs.
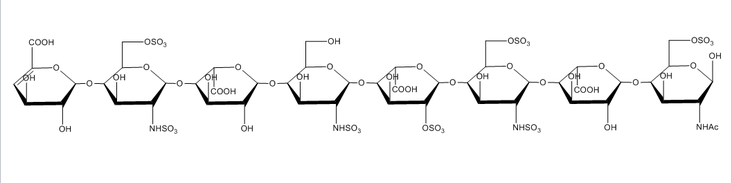
Glycosaminoglycans are long, unbranched, polydisperse polysaccharides consisting of repeating disaccharide units found on the cell surface of all mammalian cells. Glycosaminoglycans are involved in a wide variety of cell-cell, cell-protein, and cell-pathogen interactions, and the specificity of these interactions is driven by the pattern of modification of the polysaccharides, most notably N- and O-sulfation, N-acetylation, and epimerization of uronic acid residues. Because of the extreme diversity in these modifications, understanding the link between glycosaminoglycan structure and glycosaminoglycan function is a very challenging problem. Due to their almost ubiquitous role in interactions at the cell surface, our group has sought to develop new tools to meet this challenge.
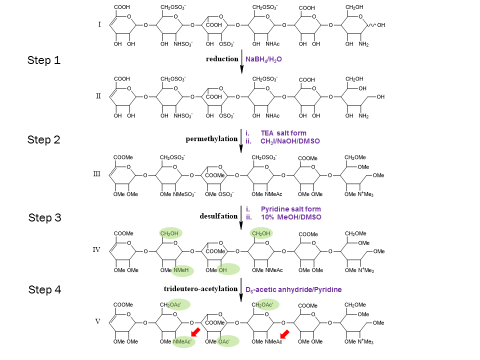
The extreme fragility of the sulfate groups on the glycosaminoglycan oligosaccharides has led us to pursue a chemical derivatization strategy to help us separate and sequence these oligosaccharides. Our approach seeks to specifically replace all labile sulfates with stable functional groups, protecting all non-sulfated N- and O-groups by permethylation. This results in an oligosaccharide that reverse-phase LC can separate, yielding diagnostic fragments by MS/MS for both sulfate position and uronic acid epimerization. Our group is actively seeking to improve the efficiency of this derivatization chemistry and improve methods for glycosaminoglycan depolymerization and oligosaccharide isolation. Ultimately, we aim to apply our tools to identify binding oligosaccharides mediating various cell surface interactions. We strive to generate or isolate drug candidates to modulate these interactions for various biomedical purposes, including anti-infective agents.
Additionally, our group is active in developing footprinting technologies to study changes in carbohydrate solvent accessible surfaces. This technology will help us identify and understand which parts of a complex carbohydrate are actually mediating interactions with protein targets, allowing us to better target interventions to modulate these crucial interactions.
